Charge of Nh3 Ligand
Why is NH3 neutral. Page 10 Page 10 Product.

Calculate The Number Of Unpaired Electrons And Lfse Of Cr Nh3 6 3 Ion Electron Configuration Crystal Field Theory Electrons
Neutral Ligands those having no negative charge on them but have an excess of electron to donate to the Central Metal.

. The main equilibrium involved in the ligand exchange reaction is. CeCuH2O62 4NH3 CuNH34H2O22 4H2Ononumber The color of. The influence of surface charge density on ligand-metal bonding is analyzed.
This is because its sigma donating capability is not very strong and. Likewise which is strongest ligand. All the halogens in group 17 F Cl Br I have a charge of -1.
Yet until this happens the presence of the lone pair makes an atom of. The lone pair of electrons can be split when the NH3 atom accepts a hydrogen proton becoming NH4 ammonium. Total charge 5 number of valence electrons 3 bonds to hydrogen 2 lone electrons 0.
Answer 1 of 9. One Nitrogen atom 1 x 3 nitrogens charge 3 Three hydrogen atoms 3 x 1 hydrogens charge 3 330 net charge of NH3 so the charge of NH3 is 0 or Neutral. The main reason for ligand.
How to determine the charge of ligands. Sulfate SO42- has a 2 charge and the only source of negative charge in the compound so the. Traditional solid-state effects such as ligand pi-pi overlap or.
Likewise what type of ligand is nh3. The ammine ligand NH3 is neutral so only the counter ions will determine the charge. Type Charge Ligand Formula Name in Complexes monodentate neutral ammonia NH3 ammine water H2O aqua carbon monoxide CO carbonyl pyridine pyr pyridine minus one azide N3.
These ligands can accept pi-electrons - Z-type ligand and donate lone pair L-type ligand synergistically making these ligands another class in itself. So if for CoNH35Cl is the ligand and it is bonded to Cl then we know Cl is Cl- with negative 1 charge and this makes the ligand positive 1 charge. NH 3 and HCOOH adsorb on the Mg 0 0 0 1 surface through the dative bond.
Therefore an atom of ammonia has zero overall charge a neutral charge. NH3 is a ligand with medium field strength. It is useful to use the periodic table.
Think of it as being one removed. The sum of the formal charges of each atom equals the overall charge of the molecule or ion. In the solid state some of these complexes exhibit split low-energy ligand-to-metal charge-transfer LMCT bands.
NH3 is a Lewis base. So if for CoNH35Cl is the ligand and it is bonded to Cl then we know Cl is Cl- with negative 1 charge and this makes the ligand positive 1 charge. Formal Charge of Hydrogen 1 valence e- 0 lone pair e- 12 x 2 bond pair e- 0.

Coordination Compounds Does N In Nh3 Ligand Gains Formal Positive Charge As N In Ammonium Ion Chemistry Stack Exchange

Coordination Compounds Does N In Nh3 Ligand Gains Formal Positive Charge As N In Ammonium Ion Chemistry Stack Exchange
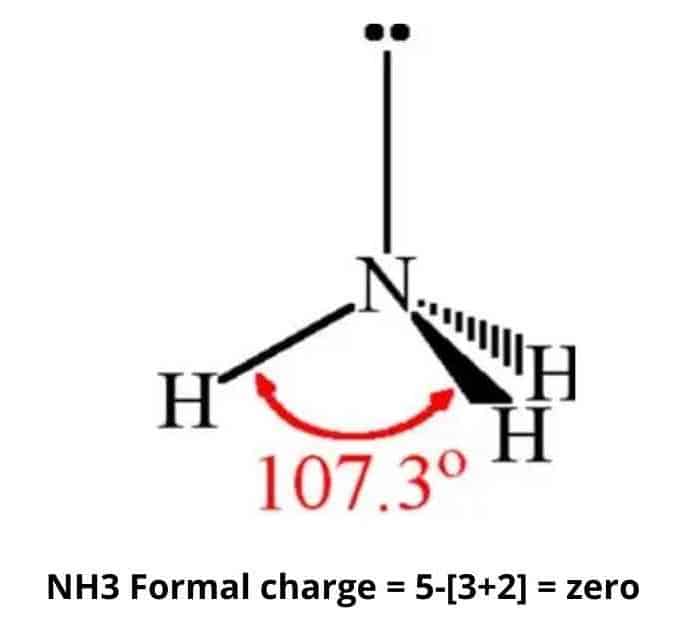
Charge Of Ammonia Nh3 Simple Steps What S Insight

Coordination Compounds Does N In Nh3 Ligand Gains Formal Positive Charge As N In Ammonium Ion Chemistry Stack Exchange
Comments
Post a Comment